Premium Information is available for this item - Upgrade for $1 a day
7530-01-435-1717
Label
7530014351717 014351717 2M9229 DPTT-OPTI54
A flexible item designed to be affixed to an object by adhesion or sewing to indicate contents, destination, or other similar identification. It may be blank with a surface designed for marking, preprinted, or have data woven into it. May be in roll form if perforations or markings are provided for detaching items of a specific length, or if separate labels are affixed to a continuous roll of backing material. Excludes MARKER, IDENTIFICATION; PLATE, IDENTIFICATION; TAPE IDENTIFICATION; and TAPE, PRESSURE SENSITIVE ADHESIVE. View more Label
![]()
January 2023
5
 Marketplace 7530-01-435-1717
Marketplace 7530-01-435-1717
Request a Quotation from participating marketplace vendors
 Related Documents 7530-01-435-1717 3+ Documents (More...)
Related Documents 7530-01-435-1717 3+ Documents (More...)
7530-01-435-1717 3+ Documents ( More... ) https//www.nsnlookup.com /fsg-75/fsc-7530/us 7530-01-435-1717
Label 7530014351717 014351717,2M9229 www.nsnlookup.com /fsg-75/fsc-7530/us 7530-01-435-1717 https
Label 7530014351717 014351717,2M9229 www.nsnlookup.com /fsg-75/fsc-7530/us 7530-01-435-1717 https
Ttr-Bxopti54Special Features [FEAT]Thermal Transfer Ribbon For Baxter (Optifill) System For Use With Dptt-Opti54
Label 7530014351717 014351717,2M9229 Baxter (Optifill) System For Use With Dptt-Opti54 https//www.nsnlookup.com
 Restrictions 7530-01-435-1717
Restrictions 7530-01-435-1717
7530-01-435-1717 is a Label that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. Demilitarization of this item has been confirmed and is not currently subject to changes. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item may be hazardous as it is in a Federal Supply Class for potentially hazardous items. A MSDS should be available from the supplier for the end user to evaluate any hazards. This item does not contain a precious metal.
 End Users 7530-01-435-1717
End Users 7530-01-435-1717
- MOE Rule:
- V540
- Effective Date:
- 1 Aug 1996
 Approved Sources 7530-01-435-1717
Approved Sources 7530-01-435-1717
- Part Number
- Manufacturer
- Status
- 2M9229
- Manufacturer
- 04687 - Baxter Healthcare Corp (Active)
- Primary Buy
- Primary Buy
- DPTT-OPTI54
- 0FB09 - Timemed Labeling Systems Inc (Active)
- Canceled/Obsolete
- Canceled/Obsolete
- DPTT-OPTI54
- 11670 - Timemed Labeling Systems, Inc. (Obsolete)
- Canceled/Obsolete
- Canceled/Obsolete
 Datasheet 7530-01-435-1717
Datasheet 7530-01-435-1717
- Characteristic
- Specifications
- FIIG
- Specifications
- A529G0
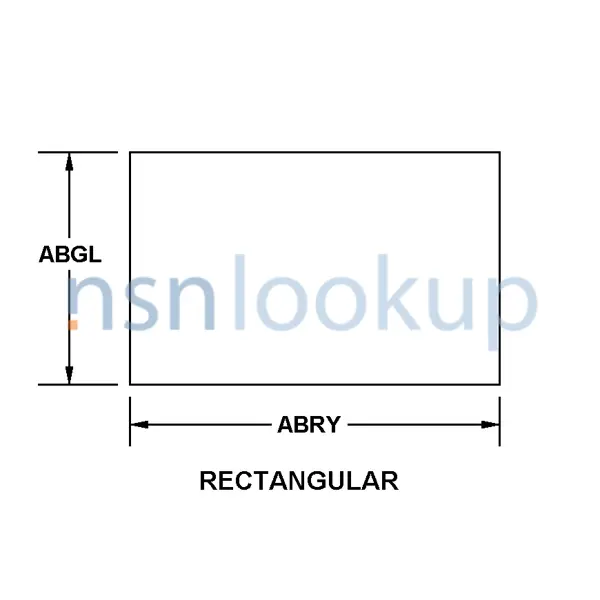
- Basic Shape Style [AGTA]
- Rectangular
- Width [ABGL]
- 4.000 Inches Nominal
- Length [ABRY]
- 5.000 Inches Nominal
- Special Features [FEAT]
- 1250 Labels Per Roll; For Baxter Opti-Fill System
 Similar Supply Items to 7530-01-435-1717
Similar Supply Items to 7530-01-435-1717


















 Freight Information 7530-01-435-1717
Freight Information 7530-01-435-1717
7530-01-435-1717 has freight characteristics.. 7530-01-435-1717 has a variance between NMFC and UFC when transported by rail and the description should be consulted.