Premium Information is available for this item - Upgrade for $1 a day
6515-01-625-3223
Endoscopic Cholangiography And Pancreatography Catheter
6515016253223 016253223 M00535700
An item designed to be used for cannulation of common bile and pancreatic ducts. This item can be used with various diameter wire guide catheters. It may have etched markings at the distal tip and a double female side arm luer lock fitting and can be autoclavable. Excludes CATHETER, CHOLANGIOGRAPHY. View more Endoscopic Cholangiography And Pancreatography Catheter
![]()
January 2023
2
 Marketplace 6515-01-625-3223
Marketplace 6515-01-625-3223
Request a Quotation from participating marketplace vendors
 Related Documents 6515-01-625-3223 5+ Documents (More...)
Related Documents 6515-01-625-3223 5+ Documents (More...)
,M00535700 -7-18- 79-LT 467-1582 6515-01 6515-01-625 6515016253223016253223,M00535700 fsc-6515/us 6515
6515016253223016253223,M00535700 013962161,94 -08-01-126 FS,GT-1 6515016253223016253223 ,M00535700
6515016253223 016253223,M00535700 013962161,94 -08-01-126 FS,GT-1 6515016253223016253223 ,M00535700
65/fsc-6515/us 6515-01-396-2161 6515013962161 013962161,94-08-01-126 FS,GT-1 6515-01-467-1582 6515-01-625-3223
6515016253223 016253223,M00535700 01-126 FS,GT-1 6515016253223016253223 ,M00535700 https//www.nsnlookup.com
6515016253223016253223,M00535700 6515016253223016253223 https// 396-2159 6515 -01-468-3369 https
6515016253223 016253223,M00535700 6515014683369 014683369 Every Day 6515-01-468-3369 https//www.nsnlookup.com
612-2956 6515-01-396-2816 6515-01-467-1582 6515-01-625-3223 https//www.nsnlookup.com /fsg-65/fsc-6515
6515016253223 016253223,M00535700 468-3369 6515014683369 014683369 Every Day 6515-01-468-3369 https
fsg-65/fsc-6515/us 6515-01-396-2816 6515013962816 013962816,94-08-01-123 FS 6515016253223016253223,M00535700
 Restrictions 6515-01-625-3223
Restrictions 6515-01-625-3223
6515-01-625-3223 is a Endoscopic Cholangiography And Pancreatography Catheter that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. Demilitarization of this item has been confirmed and is not currently subject to changes. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. This item does not contain a precious metal.
 Import and Export 6515-01-625-3223
Import and Export 6515-01-625-3223
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-01-625-3223
End Users 6515-01-625-3223
- Brazil (YA01)
- Effective Date:
- 1 Jan 2014
 Approved Sources 6515-01-625-3223
Approved Sources 6515-01-625-3223
- Part Number
- Manufacturer
- Status
- M00535700
- Manufacturer
- 0TS15 - Boston Scientific Corp Medi-Tech Div (Active)
- Primary Buy
- Primary Buy
- M00535700
- 012AK - Boston Scientific Do Brasil Ltda (Active)
- Secondary Buy
- Secondary Buy
 Datasheet 6515-01-625-3223
Datasheet 6515-01-625-3223
- Characteristic
- Specifications
- FIIG
- Specifications
- A22100
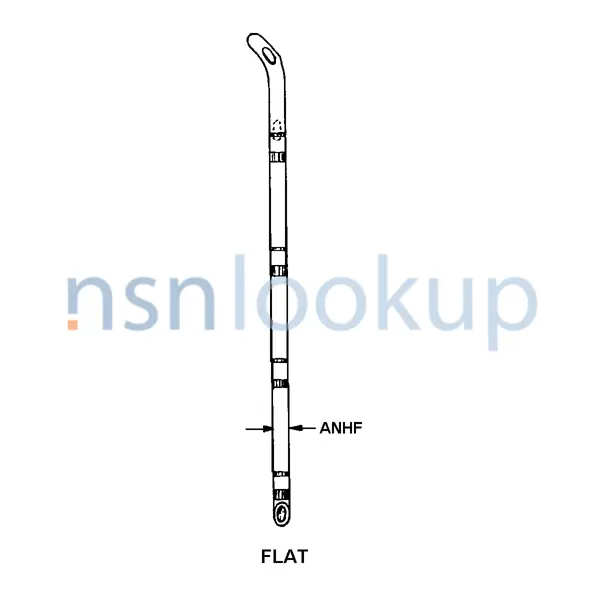
- Nominal Diameter [ANHF]
- 1.8 Millimeters And 2.3 Millimeters
- Part Name Assigned By Controlling Agency [CXCY]
- Tandem Xl Triple-Lumen Ercp Cannula
- Patient Size Designation [ANFY]
- X-Large
- Special Features [FEAT]
- Recommended Guidewire 0.89 Mm; Distal O.D. 1.83 Mm
- Tip Type [ANFG]
- Tapered
- Style Designator [STYL]
- Flat
 Similar Supply Items to 6515-01-625-3223
Similar Supply Items to 6515-01-625-3223


















 Freight Information 6515-01-625-3223
Freight Information 6515-01-625-3223
6515-01-625-3223 has freight characteristics.. 6515-01-625-3223 has a variance between NMFC and UFC when transported by rail and the description should be consulted.