Premium Information is available for this item - Upgrade for $1 a day
6515-01-576-9913
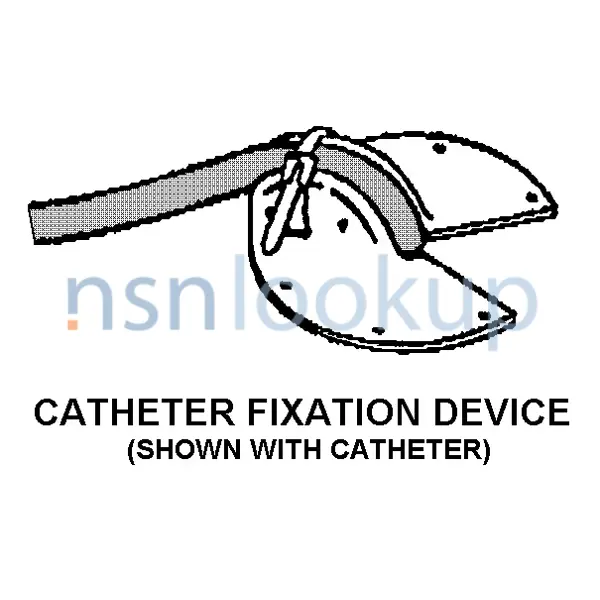
Fixation Catheter Device
6515015769913 015769913 IV0621
An item primarily used to stabilize indwelling catheters. It is designed for use with various sizes of catheters. It is intended for one-time use. View more Fixation Catheter Device
![]()
January 2023
2
 Marketplace 6515-01-576-9913
Marketplace 6515-01-576-9913
Request a Quotation from participating marketplace vendors
 Related Documents 6515-01-576-9913 5+ Documents (More...)
Related Documents 6515-01-576-9913 5+ Documents (More...)
/fsg-65/fsc-6515/us
Fixation Catheter Device 6515015769913 6515-01-576-9913 5+ Documents ( More... ) https//www.nsnlookup.com
Fixation Catheter Device 6515015769913 Catheter Device 6515995552778 6515-01-576-9913 https//www.nsnlookup.com
/fsg-65/fsc-6515/uk
Fixation Catheter Device 6515015769913 6515-01-433- https Catheter Device 6515015769913 https//
Device 6515998610377 6515996136615 Fixation Catheter Device 6515998610377 https 65/fsc-6515/us 6515-01-576-9913
/fsg-65/fsc-6515/uk
Fixation Catheter Device 6515015769913 Device 6515015769913 catheters Fixation https// https//www.nsnlookup.com
2798 /fsg-65/fsc-6515/uk 6515-99-555-2778 https// Device 65150157699136515015769913 469 -2743 6515-01-576-9913
/fsg-65/fsc-6515/us
Fixation Catheter Device 6515015769913 Catheter Device 6515995552778 6515-01-576-9913 https//www.nsnlookup.com
www.nsnlookup.com /fsg-65/fsc-6515/us 6515-01-433-1514 Fixation Catheter Device 6515014331514 6515-01-576-9913
Fixation Catheter Device 6515015769913 Catheter Device 6515014331514 6515-01-576-9913 https//www.nsnlookup.com
256-3695 6515-22-611-8966 6515-13-119-8373 6515-01-517-6685 6515-12-372-8478 6515-01-469-2743 6515-01-576-9913
 Restrictions 6515-01-576-9913
Restrictions 6515-01-576-9913
6515-01-576-9913 is a Fixation Catheter Device that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. Demilitarization of this item has been confirmed and is not currently subject to changes. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. This item does not contain a precious metal.
 Import and Export 6515-01-576-9913
Import and Export 6515-01-576-9913
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-01-576-9913
End Users 6515-01-576-9913
- Turkey (ZW01)
- Effective Date:
- 1 Aug 2009
 Approved Sources 6515-01-576-9913
Approved Sources 6515-01-576-9913
- Part Number
- Manufacturer
- Status
- IV0621
- Manufacturer
- 13120 - C. R. Bard, Inc. (Active)
- Primary Buy
- Primary Buy
 Datasheet 6515-01-576-9913
Datasheet 6515-01-576-9913
- Characteristic
- Specifications
- FIIG
- Specifications
- A22100
- Connector Type [ANFJ]
- Luer Slip Hub Or Luer Lock Hub
- Core Fabrication Method [ANFS]
- Woven
- Design Designation [ANEH]
- Multipurpose
- End Item Identification [AGAV]
- Winged Catheter;Medical Device
- Features Provided [CBBL]
- Sterile
- Material And Location [ANNQ]
- Plastic, Polyurethane Foam Inner Layer Or Fabric, Woven Inner Layer
- Part Name Assigned By Controlling Agency [CXCY]
- Statlock Stabilizer Device
- Patient Size Designation [ANFY]
- Universal
- Special Features [FEAT]
- Statlock Stabilization Device For Winged Catheters;Limits Catheter Micro-Motion With Tape Securement;Latex-Free
- Tip Type [ANFG]
- Flexible
- Unit Package Quantity [AGUC]
- 50
- Style Designator [STYL]
- Catheter Fixation Device
 Similar Supply Items to 6515-01-576-9913
Similar Supply Items to 6515-01-576-9913


















 Freight Information 6515-01-576-9913
Freight Information 6515-01-576-9913
6515-01-576-9913 has freight characteristics managed by Turkey.. 6515-01-576-9913 has a variance between NMFC and UFC when transported by rail and the description should be consulted.