Premium Information is available for this item - Upgrade for $1 a day
6515-01-458-1299
Urethral Catheter
6515014581299 014581299 0113L26
An item designed for insertion through the urinary tract as far as the bladder to allow drainage, irrigation, observation, and application of medication. For items designed to drain the ureter, see CATHETER, URETERAL. For items designed to be inserted through an incision or wound, see DRAIN, SURGICAL; and TUBE, (as modified). View more Urethral Catheter
![]()
January 2023
3
 Marketplace 6515-01-458-1299
Marketplace 6515-01-458-1299
Request a Quotation from participating marketplace vendors
 Related Documents 6515-01-458-1299 1+ Documents (More...)
Related Documents 6515-01-458-1299 1+ Documents (More...)
6515-01-458-1299 1+ Documents ( More... ) https//www.nsnlookup.com /fsg-65/fsc-6515/us 6515-01-458-1299
Day 6515-01-458-1299 RQST NE Related Documents 6515-01-458-1299 1+ Documents ( More... ) https//www.nsnlookup.com
 Restrictions 6515-01-458-1299
Restrictions 6515-01-458-1299
6515-01-458-1299 is a Urethral Catheter that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. Demilitarization of this item has been confirmed and is not currently subject to changes. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. This item does not contain a precious metal.
 Import and Export 6515-01-458-1299
Import and Export 6515-01-458-1299
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-01-458-1299
End Users 6515-01-458-1299
- MOE Rule:
- V540
- Effective Date:
- 1 Aug 1998
 Approved Sources 6515-01-458-1299
Approved Sources 6515-01-458-1299
- Part Number
- Manufacturer
- Status
- 0113L26
- Manufacturer
- 2P778 - Bard C R Inc Bard Urological Div (Active)
- Primary Buy
- Primary Buy
 Datasheet 6515-01-458-1299
Datasheet 6515-01-458-1299
- Characteristic
- Specifications
- FIIG
- Specifications
- A22100
- Style Designator [STYL]
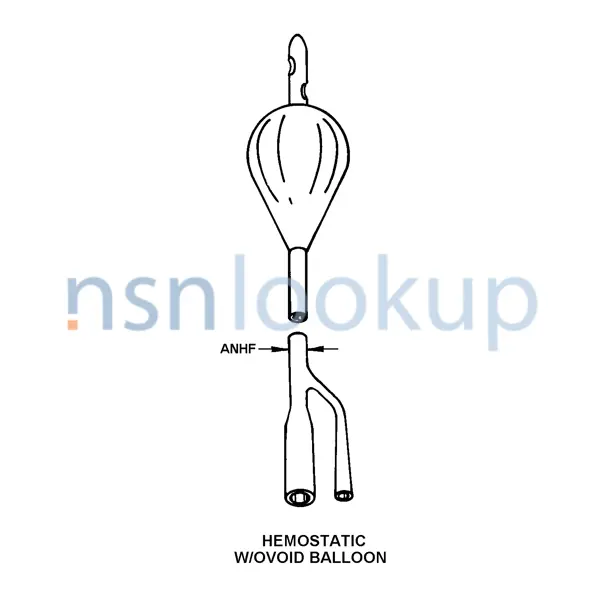
- Hemostatic W/ Ovoid Balloon
- Design Designation [ANEH]
- Foley
- Nominal Diameter [ANHF]
- 26.000 French
- Material And Location [ANNQ]
- Rubber, Latex Overall
- Shaft Eye Quantity [ANER]
- 2
- Balloon Capacity [ANES]
- 75.0 Cubic Centimeters
- Inflation Device [ANFC]
- Self-Sealing Valve
- Tip Type [ANFG]
- Round
- Unit Package Quantity [AGUC]
- 12
- Features Provided [CBBL]
- Sterile
- Special Features [FEAT]
- Ovoid Fluted Balloon, Lubricious Catheter, Medium Length, Round Tip With 2 Staggered Eyes, Extra-Strong Preformed Ovoid Balloon For Post-Operative Hemostasis
 Similar Supply Items to 6515-01-458-1299
Similar Supply Items to 6515-01-458-1299


















 Freight Information 6515-01-458-1299
Freight Information 6515-01-458-1299
6515-01-458-1299 has freight characteristics.. 6515-01-458-1299 has a variance between NMFC and UFC when transported by rail and the description should be consulted.