Premium Information is available for this item - Upgrade for $1 a day
6515-01-455-1039
Thoracic Catheter
6515014551039 014551039 DRAC-16S
An item with an open tip and connector end designed for use in drainage of chest wounds. View more Thoracic Catheter
![]()
January 2023
4
 Marketplace 6515-01-455-1039
Marketplace 6515-01-455-1039
Request a Quotation from participating marketplace vendors
 Related Documents 6515-01-455-1039 5+ Documents (More...)
Related Documents 6515-01-455-1039 5+ Documents (More...)
6515-01-455-1039 5+ Documents ( More... ) https//www.nsnlookup.com /fsg-65/fsc-6515/us 6515-01-455-1039
Day 6515-01-455-1039 RQST NE Related Documents 6515-01-455-1039 5+ Documents ( More... ) https//www.nsnlookup.com
www.nsnlookup.com /fsg-65/fsc-6515/uk 6515-99-257-9635 https//www.nsnlookup.com /fsg-65/fsc-6515/us 6515-01-455-1039
Thoracic Catheter 6515014551039 014551039 Documents ( More... ) https//www.nsnlookup.com https/
Thoracic Catheter 6515014551039 014551039 Catheter 6515014550935 014550935 https//www.nsnlookup.com
-6515/tr 6515-27-027-1146 Thoracic Catheter 6515270271146270271146 027-1146 https 940 -6058 6515-01-455-1039
Thoracic Catheter 6515014551039 014551039 www.nsnlookup.com https/ fsg-65/fsc-6515/us 6515-01-455
-6515/tr 6515-27-027-1146 Thoracic Catheter 6515270271146 270271146 027-1146 https 940 -6058 6515-01-455-1039
Thoracic Catheter 6515014551039 014551039 Documents ( More... ) https//www.nsnlookup.com https/
fsc-6515/us 6515-01-156-2733 https Catheter 6515014551039014551039 6515-27-027-1146 940-6058 6515-01-455-1039
 Restrictions 6515-01-455-1039
Restrictions 6515-01-455-1039
6515-01-455-1039 is a Thoracic Catheter that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. Demilitarization of this item has been confirmed and is not currently subject to changes. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. This item does not contain a precious metal.
 Import and Export 6515-01-455-1039
Import and Export 6515-01-455-1039
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-01-455-1039
End Users 6515-01-455-1039
- MOE Rule:
- V540
- Effective Date:
- 1 Apr 1998
 Approved Sources 6515-01-455-1039
Approved Sources 6515-01-455-1039
- Part Number
- Manufacturer
- Status
- DRAC-16S
- Manufacturer
- 1CXU4 - Genzyme Surgical Products (Active)
- Primary Buy
- Primary Buy
 Datasheet 6515-01-455-1039
Datasheet 6515-01-455-1039
- Characteristic
- Specifications
- FIIG
- Specifications
- A22100
- Design Designation [ANEH]
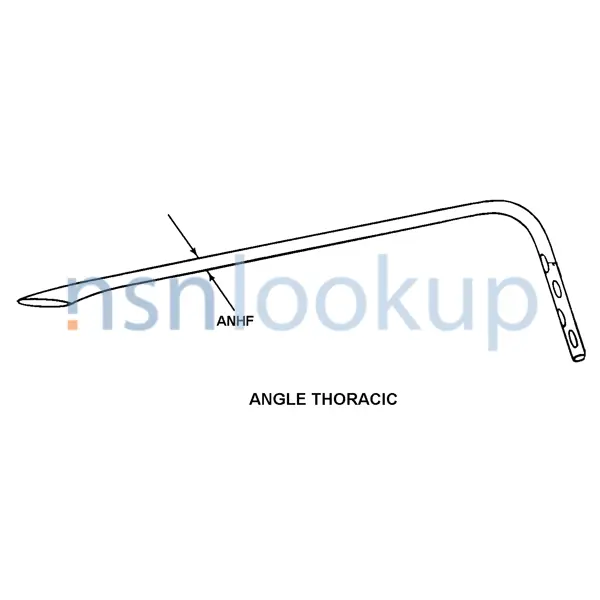
- Thoracic
- Features Provided [CBBL]
- Depth Graduation Markings And Disposable And Sterile And X-Ray Opacity
- Nominal Diameter [ANHF]
- 16.000 French
- Inside Diameter [AARX]
- 0.140 Inches Nominal
- Material And Location [ANNQ]
- Plastic, Polyvinyl Chloride Overall
- Shaft Eye Quantity [ANER]
- 6
- Overall Length [ABHP]
- 21.000 Inches Nominal
- Special Features [FEAT]
- Soft; Sealed Blue Tip; Right Angle
- Tip Type [ANFG]
- Closed
- Unit Package Quantity [AGUC]
- 10
- Style Designator [STYL]
- Angle Thoracic
 Similar Supply Items to 6515-01-455-1039
Similar Supply Items to 6515-01-455-1039


















 Freight Information 6515-01-455-1039
Freight Information 6515-01-455-1039
6515-01-455-1039 has freight characteristics.. 6515-01-455-1039 has a variance between NMFC and UFC when transported by rail and the description should be consulted.