Premium Information is available for this item - Upgrade for $1 a day
6515-01-392-0171
Surgical Absorbable Suture
6515013920171 013920171 J936H
An item designed for stitching and thus securing tissues. It is designed of a material which will be dissolved and absorbed in such tissues. View more Surgical Absorbable Suture
![]()
January 2023
2
 Marketplace 6515-01-392-0171
Marketplace 6515-01-392-0171
Request a Quotation from participating marketplace vendors
 Related Documents 6515-01-392-0171 1+ Documents (More...)
Related Documents 6515-01-392-0171 1+ Documents (More...)
6515-01-392-0171 1+ Documents ( More... ) https//www.nsnlookup.com /fsg-65/fsc-6515/us 6515-01-392-0171
Suture 6515013920171 RQST NE Updated Every Day 6515-01-392-0171 RQST Updated Every /fsg-65/fsc-6515/
 Restrictions 6515-01-392-0171
Restrictions 6515-01-392-0171
6515-01-392-0171 is a Surgical Absorbable Suture that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. This item does not contain a precious metal.
 Import and Export 6515-01-392-0171
Import and Export 6515-01-392-0171
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-01-392-0171
End Users 6515-01-392-0171
- MOE Rule:
- V542
- Effective Date:
- 1 Jun 1994
 Approved Sources 6515-01-392-0171
Approved Sources 6515-01-392-0171
- Part Number
- Manufacturer
- Status
- J936H
- Manufacturer
- 10614 - Ethicon, Inc (Obsolete)
- Original Design
- Original Design
 Datasheet 6515-01-392-0171
Datasheet 6515-01-392-0171
- Characteristic
- Specifications
- FIIG
- Specifications
- A21900
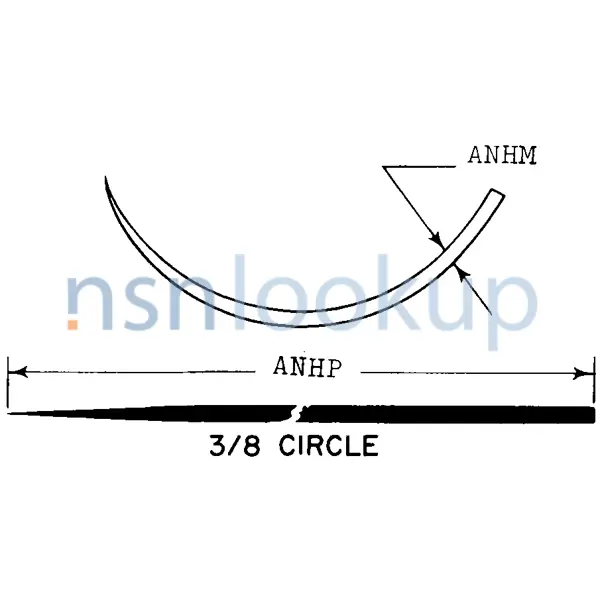
- Armed Suture Needle Style [APWL]
- 3/8 Circle
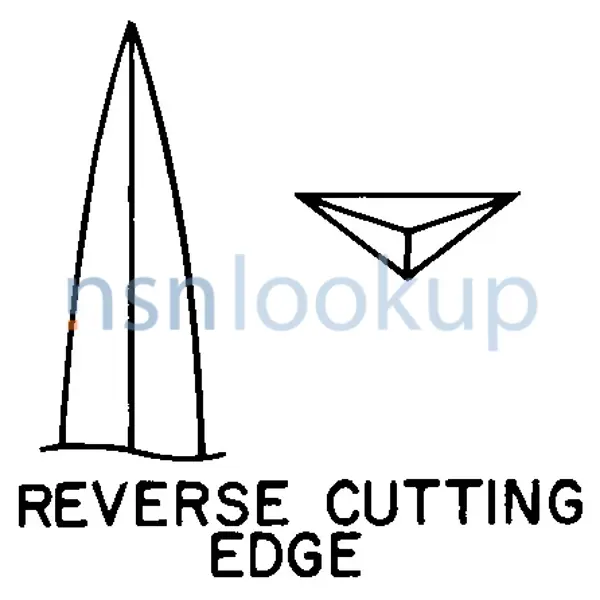
- Armed Suture Needle Point Style [APRH]
- Reverse Cutting Edge
- Color [HUES]
- Natural
- End Type [APTD]
- Single Armed
- Needle Design Designation [APSH]
- Plastic Surgery
- Immediate Container Quantity [ANNX]
- 36
- Immediate Container Type [ANNW]
- Packet, Foil
- Length [ABRY]
- 27.000 Inches Nominal
- Material And Location [ANNQ]
- Plastic, Glycolide-Lactide Copolymer Suture And Steel, Corrosion Resisting Needle
- Quantity Within Each Immediate Container [ANNY]
- 1
- Size Designator [AJXE]
- 3-0
- Special Features [FEAT]
- Coated Vicryl; Precision Point; Needle, Ps-1
- Unit Package Quantity [AGUC]
- 36
- Sterility [AKMX]
- Sterile
- Strand Fiber Arrangement [APZJ]
- Braided
 Management Data 6515-01-392-0171
Management Data 6515-01-392-0171
- Effective Date
- Organization
- Unit of Issue
- Unit Price
- Qty Unit Pack
- Unit of Issue
- Jun 1994
- Veterans Administration (VA)
- DZ
- Subscribe
- 1
- DZ
 Similar Supply Items to 6515-01-392-0171
Similar Supply Items to 6515-01-392-0171


















 Freight Information 6515-01-392-0171
Freight Information 6515-01-392-0171
6515-01-392-0171 has freight characteristics.. 6515-01-392-0171 has a variance between NMFC and UFC when transported by rail and the description should be consulted.