Premium Information is available for this item - Upgrade for $1 a day
6515-01-368-2859
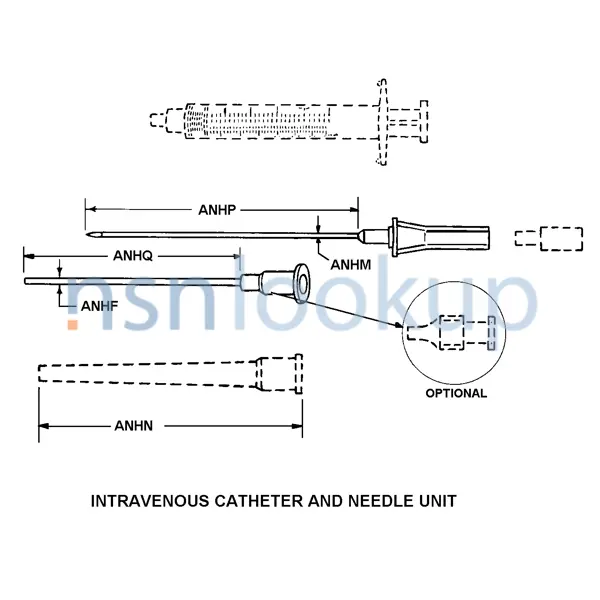
Intravenous Catheter And Needle Unit
6515013682859 013682859 F92G011 92-7-15-45 FS 38-8324-1
An item consisting of a cannulated needle with an intravenous catheter, and it may contain additional components, such as a hypodermic syringe, needle guard, flow control plug, and the like. The catheter is inserted into the vein by way of the needle's puncture, and is left in position for continued intravenous administration after the needle is withdrawn. A syringe, when supplied, is used to better control insertion of the catheter needle unit or for blood aspiration. View more Intravenous Catheter And Needle Unit
![]()
January 2023
4
 Marketplace 6515-01-368-2859
Marketplace 6515-01-368-2859
Request a Quotation from participating marketplace vendors
 Related Documents 6515-01-368-2859 1+ Documents (More...)
Related Documents 6515-01-368-2859 1+ Documents (More...)
RQST NE Updated Every Day 6515-01-368-2859 RQST Updated Every Day 6515-01-368-2859 RQST NE Related
Documents 6515-01-368-2859 1+ Documents ( More... ) https//www.nsnlookup.com /fsg-65/fsc-6515/us 6515
 Restrictions 6515-01-368-2859
Restrictions 6515-01-368-2859
6515-01-368-2859 is a Intravenous Catheter And Needle Unit that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. This item does not contain a precious metal.
 Import and Export 6515-01-368-2859
Import and Export 6515-01-368-2859
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-01-368-2859
End Users 6515-01-368-2859
- MOE Rule:
- V542
- Effective Date:
- 1 Apr 1991
 Approved Sources 6515-01-368-2859
Approved Sources 6515-01-368-2859
- Part Number
- Manufacturer
- Status
- 38-8324-1
- Manufacturer
- 31202 - Becton Dickinson And Co (Active)
- Primary Buy
- Primary Buy
- F92G011
- 66735 - Air Force Medical Logistics Office (Active)
- Incomplete Secondary Reference
- Incomplete Secondary Reference
- 92-7-15-45 FS
- 66735 - Air Force Medical Logistics Office (Active)
- Incomplete Secondary Reference
- Incomplete Secondary Reference
 Datasheet 6515-01-368-2859
Datasheet 6515-01-368-2859
- Characteristic
- Specifications
- FIIG
- Specifications
- A22100
- Catheter Nominal Length [ANHQ]
- 0.7 Millimeters
- Design Designation [ANEH]
- Intravenous
- Features Provided [CBBL]
- Sterile And Disposable
- Needle Nominal Diameter [ANHM]
- 24.000 Gage
- Needle Nominal Length [ANHP]
- 0.750 Millimeters
- Nominal Diameter [ANHF]
- 1.9 Centimeters
- Material And Location [ANNQ]
- Steel, Corrosion Resisting Overall
- Special Features [FEAT]
- Color Code Lavender; Individually Packaged
- Tip Type [ANFG]
- Open End
- Unit Package Quantity [AGUC]
- 200
- Style Designator [STYL]
- Intravenous Catheter And Needle Unit
 Management Data 6515-01-368-2859
Management Data 6515-01-368-2859
- Effective Date
- Organization
- Unit of Issue
- Unit Price
- Qty Unit Pack
- Unit of Issue
- May 1993
- Veterans Administration (VA)
- PG
- Subscribe
- PG
- MOE
- USC
- Code
- Statement
- Order of Use
- Jump To Code
- Qty Per Assy
- UOM
- Technical Document
- Quantative Expression
- Code
- Veterans Administration (VA)
- V
- K
- U/I Contains 200 Ea
- 00000000200EA
- K
 Similar Supply Items to 6515-01-368-2859
Similar Supply Items to 6515-01-368-2859


















 Freight Information 6515-01-368-2859
Freight Information 6515-01-368-2859
6515-01-368-2859 has freight characteristics managed by the Bonneville Power Administration.. 6515-01-368-2859 has a variance between NMFC and UFC when transported by rail and the description should be consulted.