Premium Information is available for this item - Upgrade for $1 a day
6515-01-356-0235
Surgical Wound Evacuator Tube
6515013560235 013560235 92-1-22-13-LT A92A167 3461
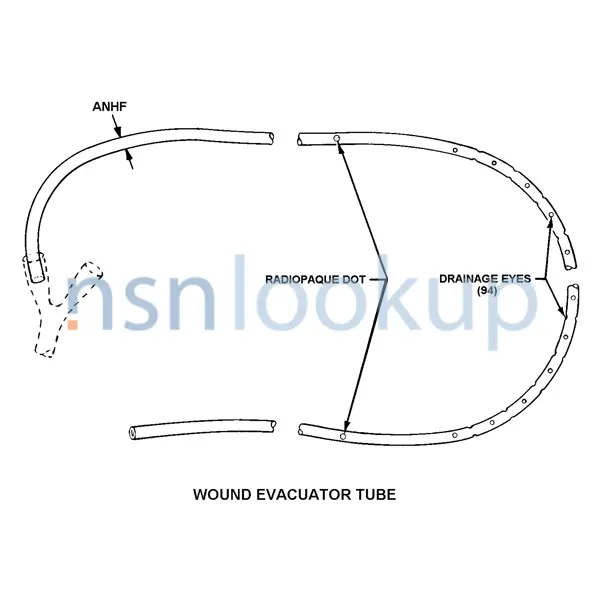
A tubular item used with an EVACUATOR, SURGICAL WOUND that is designed to be left inside a surgical incision, after closure, to facilitate in suction removal of post-operative liquification. View more Surgical Wound Evacuator Tube
![]()
January 2023
5
 Marketplace 6515-01-356-0235
Marketplace 6515-01-356-0235
Request a Quotation from participating marketplace vendors
 Related Documents 6515-01-356-0235 1+ Documents (More...)
Related Documents 6515-01-356-0235 1+ Documents (More...)
RQST NE Updated Every Day 6515-01-356-0235 RQST Updated Every Day 6515-01-356-0235 RQST NE Related
Documents 6515-01-356-0235 1+ Documents ( More... ) https//www.nsnlookup.com /fsg-65/fsc-6515/us 6515
 Restrictions 6515-01-356-0235
Restrictions 6515-01-356-0235
6515-01-356-0235 is a Surgical Wound Evacuator Tube that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. This item does not contain a precious metal.
 Import and Export 6515-01-356-0235
Import and Export 6515-01-356-0235
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-01-356-0235
End Users 6515-01-356-0235
- MOE Rule:
- V540
- Effective Date:
- 1 Mar 1997
 Approved Sources 6515-01-356-0235
Approved Sources 6515-01-356-0235
- Part Number
- Manufacturer
- Status
- 3461
- Manufacturer
- 6V556 - United Medical Supply Po Box 219 (Obsolete)
- Primary Buy
- Primary Buy
- 92-1-22-13-LT
- 66732 - Department Of The Army U S Army (Active)
- Incomplete Secondary Reference
- Incomplete Secondary Reference
- A92A167
- 66732 - Department Of The Army U S Army (Active)
- Incomplete Secondary Reference
- Incomplete Secondary Reference
 Datasheet 6515-01-356-0235
Datasheet 6515-01-356-0235
- Characteristic
- Specifications
- FIIG
- Specifications
- A22100
- Core Fabrication Method [ANFS]
- Not Woven
- Design Designation [ANEH]
- Wound Drainage
- Features Provided [CBBL]
- Flexible And Sterile And Disposable And X-Ray Opacity
- Nominal Diameter [ANHF]
- 12.500 Inches
- Material And Location [ANNQ]
- Plastic, Polyvinyl Chloride Overall And Silicone Rubber Overall
- Patient Size Designation [ANFY]
- Adult And Medium
- Special Features [FEAT]
- Reliavac 400 Cc; Y-Connecting Tube; Anti-Reflux Valve
- Tip Type [ANFG]
- Open End
- Unit Package Quantity [AGUC]
- 10
- Style Designator [STYL]
- Wound Evacuator Tube
 Similar Supply Items to 6515-01-356-0235
Similar Supply Items to 6515-01-356-0235


















 Freight Information 6515-01-356-0235
Freight Information 6515-01-356-0235
6515-01-356-0235 has freight characteristics managed by the Bonneville Power Administration.. 6515-01-356-0235 has a variance between NMFC and UFC when transported by rail and the description should be consulted.