Premium Information is available for this item - Upgrade for $1 a day
6515-01-041-9812
Urethral Catheter
6515010419812 010419812 605189 4012 8887-605189
An item designed for insertion through the urinary tract as far as the bladder to allow drainage, irrigation, observation, and application of medication. For items designed to drain the ureter, see CATHETER, URETERAL. For items designed to be inserted through an incision or wound, see DRAIN, SURGICAL; and TUBE, (as modified). View more Urethral Catheter
![]()
January 2023
7
 Marketplace 6515-01-041-9812
Marketplace 6515-01-041-9812
Request a Quotation from participating marketplace vendors
 Related Documents 6515-01-041-9812 5+ Documents (More...)
Related Documents 6515-01-041-9812 5+ Documents (More...)
Documents 6515-00-104-8695 5+ Documents ( More... ) https//www.nsnlookup.com /fsg-65/fsc-6515/us 6515-01-041-9812
Urethral Catheter 6515010419812 010419812 Upgrade your Account Upgrade 6515-01-041-9812Urethral
Urethral Catheter 6515010419812 010419812 9812 https//www.nsnlookup.com /cage The Kendall Company
 Restrictions 6515-01-041-9812
Restrictions 6515-01-041-9812
6515-01-041-9812 is a Urethral Catheter that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. The precious metals content of this item is unknown.
 Import and Export 6515-01-041-9812
Import and Export 6515-01-041-9812
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-01-041-9812
End Users 6515-01-041-9812
- MOE Rule:
- V540
- Effective Date:
- 1 Apr 1991
 Approved Sources 6515-01-041-9812
Approved Sources 6515-01-041-9812
- Part Number
- Manufacturer
- Status
- 605189
- Manufacturer
- 05963 - Sherwood Medical Co (Obsolete)
- Primary Buy
- Primary Buy
- 4012
- 06135 - The Kendall Company Lp (Replaced)
- Canceled/Obsolete
- Canceled/Obsolete
- 8887-605189
- 05963 - Sherwood Medical Co (Obsolete)
- Canceled/Obsolete
- Canceled/Obsolete
- 4012
- 6VKK4 - Covidien Sales Llc (Active)
- Advisory Reference
- Advisory Reference
 Datasheet 6515-01-041-9812
Datasheet 6515-01-041-9812
- Characteristic
- Specifications
- FIIG
- Specifications
- A22100
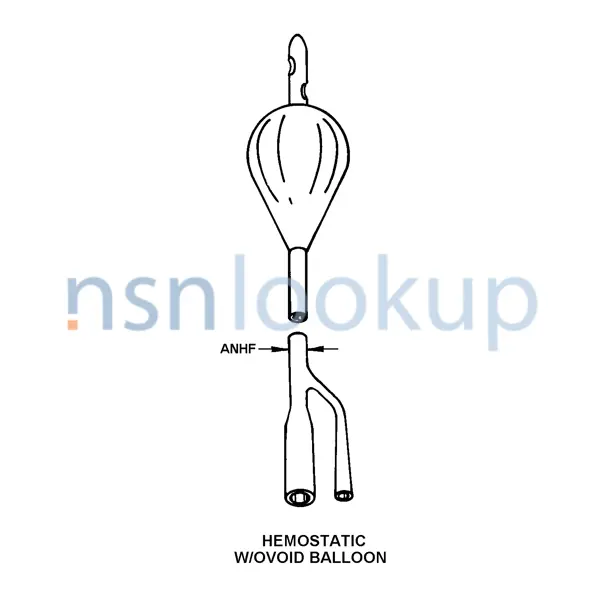
- Balloon Capacity [ANES]
- 5.0 Cubic Centimeters
- Design Designation [ANEH]
- Foley
- Features Provided [CBBL]
- Sterile And Disposable
- Nominal Diameter [ANHF]
- 18.000 French
- Inflation Device [ANFC]
- Valve
- Material And Location [ANNQ]
- Rubber Catheter
- Shaft Eye Quantity [ANER]
- 2
- Tip Type [ANFG]
- Round
- Style Designator [STYL]
- Hemostatic W/ Ovoid Balloon
 NATO Stock Numbers Related to 6515-01-041-9812
NATO Stock Numbers Related to 6515-01-041-9812
 Freight Information 6515-01-041-9812
Freight Information 6515-01-041-9812
6515-01-041-9812 has freight characteristics.. 6515-01-041-9812 has a variance between NMFC and UFC when transported by rail and the description should be consulted.