Premium Information is available for this item - Upgrade for $1 a day
6515-01-009-3017
Intravenous Catheter And Needle Unit
6515010093017 010093017 HR18003-004374
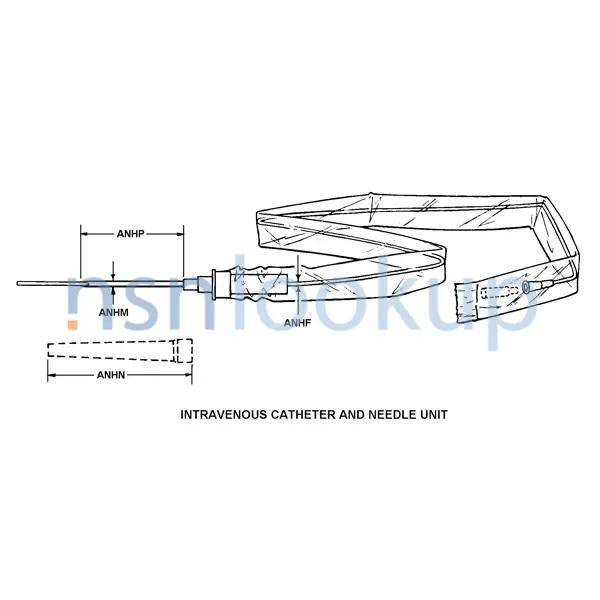
An item consisting of a cannulated needle with an intravenous catheter, and it may contain additional components, such as a hypodermic syringe, needle guard, flow control plug, and the like. The catheter is inserted into the vein by way of the needle's puncture, and is left in position for continued intravenous administration after the needle is withdrawn. A syringe, when supplied, is used to better control insertion of the catheter needle unit or for blood aspiration. View more Intravenous Catheter And Needle Unit
![]()
January 2023
11
 Marketplace 6515-01-009-3017
Marketplace 6515-01-009-3017
Request a Quotation from participating marketplace vendors
 Related Documents 6515-01-009-3017 4+ Documents (More...)
Related Documents 6515-01-009-3017 4+ Documents (More...)
6515-01-009-3017 4+ Documents ( More... ) https//www.nsnlookup.com /fsg-65/fsc-6515/us 6515-01-009-3017
6515-01-009-3017 RQST NE Related Documents 6515-01-009-3017 4+ Documents ( More... ) https//www.nsnlookup.com
And Needle Unit Account Upgrade 6515-01-009-3017Intravenous Catheter And Needle Unit6515010093017 010093017
HR18003-004374 Valve Disk 4820011818701 011818701,0043474-3 3004374004374-3 An item of various shapes
And Needle Unit Account Upgrade 6515-01-009-3017Intravenous Catheter And Needle Unit6515010093017 010093017
HR18003-004374 Valve Disk 4820011818701 011818701,0043474-3 5041Transmitter Test Set6625008305041 008305041
6625008305041008305041 5041 Transmitter Test Set 6625008305041008305041 https Needle Unit6515010093017 010093017
HR18003-004374 https//www.nsnlookup.com /fsg-66/fsc-6625/us 6625-00-830-5041 https//www.nsnlookup.com
 Restrictions 6515-01-009-3017
Restrictions 6515-01-009-3017
6515-01-009-3017 is a Intravenous Catheter And Needle Unit that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. Demilitarization of this item has been confirmed and is not currently subject to changes. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. This item does not contain a precious metal.
 Import and Export 6515-01-009-3017
Import and Export 6515-01-009-3017
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-01-009-3017
End Users 6515-01-009-3017
- Portugal (ZP)
- Portugal (ZP01)
- Effective Date:
- 1 Apr 1991
 Approved Sources 6515-01-009-3017
Approved Sources 6515-01-009-3017
- Part Number
- Manufacturer
- Status
- HR18003-004374
- Manufacturer
- 25650 - Critikon Inc (Obsolete)
- Original Design
- Original Design
 Datasheet 6515-01-009-3017
Datasheet 6515-01-009-3017
- Characteristic
- Specifications
- FIIG
- Specifications
- A22100
- Connector Type [ANFJ]
- Luer Lock Hub
- Core Fabrication Method [ANFS]
- Not Woven
- Design Designation [ANEH]
- Intravenous
- Features Provided [CBBL]
- Sterile And Disposable And X-Ray Opacity
- Needle Guard Nominal Length [ANHN]
- 3.275 Inches
- Needle Nominal Diameter [ANHM]
- 18.000 Gage
- Needle Nominal Length [ANHP]
- 2.500 Inches
- Nominal Diameter [ANHF]
- 18.000 Gage
- Material And Location [ANNQ]
- Plastic, Polytetrafluoroethylene Catheter And Steel, Corrosion Resisting Needle And Plastic Needle Guard And Plastic Hub And Plastic Plug And Plastic Sleeve And Plastic Stylet And Plastic Valve
- Special Features [FEAT]
- W/Radiopaque Polytetrafluoroethylene Introducing Outer Catheter 16.000 Gage,2.500 In. Lg,And Radiopaque Central Venous Pressure Monitoring Inner Catheter 26.000 In. Lg
- Tip Type [ANFG]
- Beveled
- Style Designator [STYL]
- Intravenous Catheter And Needle Unit
 Similar Supply Items to 6515-01-009-3017
Similar Supply Items to 6515-01-009-3017


















 Freight Information 6515-01-009-3017
Freight Information 6515-01-009-3017
6515-01-009-3017 has freight characteristics managed by the Bonneville Power Administration.. 6515-01-009-3017 has a variance between NMFC and UFC when transported by rail and the description should be consulted.