Premium Information is available for this item - Upgrade for $1 a day
6515-00-348-4800
Hypodermic Needle
6515003484800 003484800 1363 7505
A cannulated item used for aspirating or injecting fluids into body tissue. View more Hypodermic Needle
![]()
January 2023
10
 Marketplace 6515-00-348-4800
Marketplace 6515-00-348-4800
Request a Quotation from participating marketplace vendors
 Related Documents 6515-00-348-4800 5+ Documents (More...)
Related Documents 6515-00-348-4800 5+ Documents (More...)
Request a Quotation from participating marketplace vendors Request Updated Every Day 1005-27-005-7505
RQST NE Updated Every Day 1005-27-005-7505 RQST Updated Every Day 1005-27-005-7505 RQST NE Restrictions
Request a Quotation from participating marketplace vendors Request Updated Every Day 5342-33-161-1363
RQST NE Updated Every Day 5342-33-161-1363 RQST Updated Every Day 5342-33-161-1363 RQST NE Restrictions
Request a Quotation from participating marketplace vendors Request Updated Every Day 8415-22-626-1363
RQST NE Updated Every Day 8415-22-626-1363 RQST Updated Every Day 8415-22-626-1363 RQST NE Related Documents
 Restrictions 6515-00-348-4800
Restrictions 6515-00-348-4800
6515-00-348-4800 is a Hypodermic Needle that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. Demilitarization of this item has been confirmed and is not currently subject to changes. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. The precious metals content of this item is unknown.
 Import and Export 6515-00-348-4800
Import and Export 6515-00-348-4800
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-00-348-4800
End Users 6515-00-348-4800
- MOE Rule:
- V542
- Effective Date:
- 1 Apr 1991
- Brazil (YA01)
- Effective Date:
- 1 Dec 2006
- Turkey (ZW01)
- Effective Date:
- 1 Nov 1998
 Approved Sources 6515-00-348-4800
Approved Sources 6515-00-348-4800
- Part Number
- Manufacturer
- Status
- 1363
- Manufacturer
- 06531 - Becton, Dickinson And Company (Active)
- Primary Buy
- Primary Buy
- 7505
- 95388 - Popper & Sons, Inc. (Active)
- Primary Buy
- Primary Buy
 Datasheet 6515-00-348-4800
Datasheet 6515-00-348-4800
- Characteristic
- Specifications
- FIIG
- Specifications
- A21800
- Cannula Bevel Designation [ANNP]
- Regular
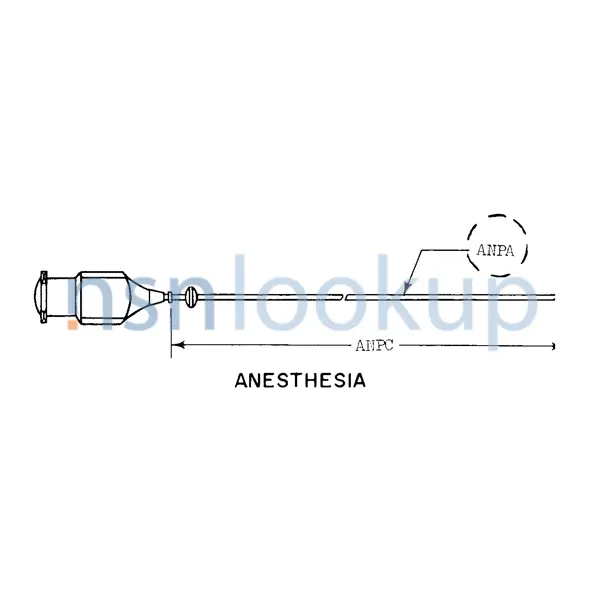
- Cannula Length [ANPC]
- 1.500 Inches Nominal
- Cannula Wall Thickness Designation [ANNN]
- Regular
- Hub Type [ANNM]
- Luer Lock
- Needle Cannula Gage Designation And Location [ANNS]
- 22 Gage Cannula
- Nondefinitive Spec/Std Data [ZZZT]
- 1 Type And A Point Style And 2 Hub Style
- Material And Location [ANNQ]
- Brass Hub
- Material And Location [ANNQ]
- Steel, Corrosion Resisting Cannula
- Sterility [AKMX]
- Unable To Decode
- Disposition After Initial Use [ANGD]
- Unable To Decode
- Unit Package Quantity [AGUC]
- 12
- Style Designator [STYL]
- Anesthesia
- Surface Treatment And Location [ANNR]
- Chromium Plated Hub
 Management Data 6515-00-348-4800
Management Data 6515-00-348-4800
- Effective Date
- Organization
- Unit of Issue
- Unit Price
- Qty Unit Pack
- Unit of Issue
- Sep 1988
- Veterans Administration (VA)
- EA
- Subscribe
- EA
 NATO Stock Numbers Related to 6515-00-348-4800
NATO Stock Numbers Related to 6515-00-348-4800
 Freight Information 6515-00-348-4800
Freight Information 6515-00-348-4800
6515-00-348-4800 has freight characteristics.. 6515-00-348-4800 has a variance between NMFC and UFC when transported by rail and the description should be consulted.