Premium Information is available for this item - Upgrade for $1 a day
6515-00-176-5465
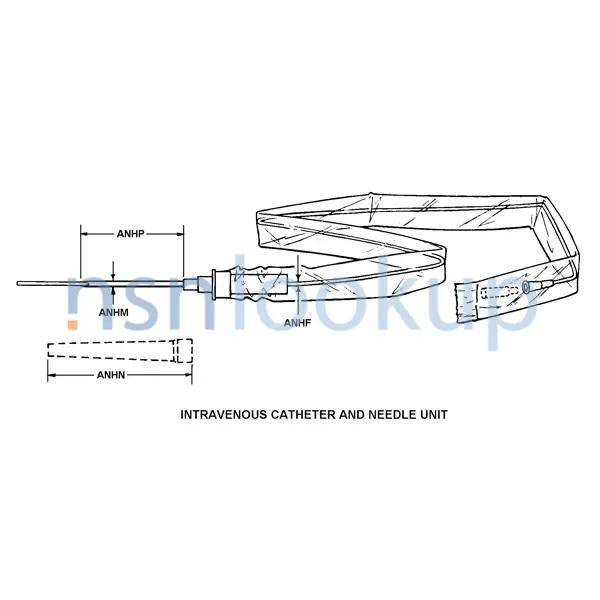
Intravenous Catheter And Needle Unit
6515001765465 001765465 3174
An item consisting of a cannulated needle with an intravenous catheter, and it may contain additional components, such as a hypodermic syringe, needle guard, flow control plug, and the like. The catheter is inserted into the vein by way of the needle's puncture, and is left in position for continued intravenous administration after the needle is withdrawn. A syringe, when supplied, is used to better control insertion of the catheter needle unit or for blood aspiration. View more Intravenous Catheter And Needle Unit
![]()
January 2023
8
 Marketplace 6515-00-176-5465
Marketplace 6515-00-176-5465
Request a Quotation from participating marketplace vendors
 Related Documents 6515-00-176-5465 2+ Documents (More...)
Related Documents 6515-00-176-5465 2+ Documents (More...)
6515-00-176-5465 1+ Documents ( More... ) https//www.nsnlookup.com /fsg-65/fsc-6515/us 6515-00-176-5465
Request Global Test Supply 3174 Hioki 3174 6515-00-176-5465 1+ Documents ( More... ) https//www.nsnlookup.com
 Restrictions 6515-00-176-5465
Restrictions 6515-00-176-5465
6515-00-176-5465 is a Intravenous Catheter And Needle Unit that does not have a nuclear hardened feature or any other critical feature such as tolerance, fit restriction or application. This item is considered a low risk when released from the control of the Department of Defense. The item may still be subject to the requirements of the Export Administration Regulations (EAR) and the Code of Federal Regulations (CFR). This item is not suspected to be hazardous. The precious metals content of this item is unknown.
 Import and Export 6515-00-176-5465
Import and Export 6515-00-176-5465
- Schedule B
- Subscribe to View Schedule B
- HTS Code
- Subscribe to View HTS Code
 End Users 6515-00-176-5465
End Users 6515-00-176-5465
- MOE Rule:
- V542
- Effective Date:
- 1 Apr 1991
 Approved Sources 6515-00-176-5465
Approved Sources 6515-00-176-5465
- Part Number
- Manufacturer
- Status
- 3174
- Manufacturer
- 31202 - Becton Dickinson And Co (Active)
- Primary Buy
- Primary Buy
 Datasheet 6515-00-176-5465
Datasheet 6515-00-176-5465
- Characteristic
- Specifications
- FIIG
- Specifications
- A22100
- Connector Type [ANFJ]
- Slip Hub
- Core Fabrication Method [ANFS]
- Not Woven
- Design Designation [ANEH]
- Intravenous
- Features Provided [CBBL]
- Sterile And Disposable And X-Ray Opacity
- Needle Nominal Diameter [ANHM]
- 0.059 Inches
- Needle Nominal Length [ANHP]
- 2.000 Inches
- Nominal Diameter [ANHF]
- 0.042 Inches
- Material And Location [ANNQ]
- Steel, Corrosion Resisting Needle And Plastic Catheter And Plastic Sleeve
- Special Features [FEAT]
- 12 In.L,Wire Stylet,Color Coded Green
- Style Designator [STYL]
- Intravenous Catheter And Needle Unit
- Tip Type [ANFG]
- Open End
 Management Data 6515-00-176-5465
Management Data 6515-00-176-5465
- Effective Date
- Organization
- Unit of Issue
- Unit Price
- Qty Unit Pack
- Unit of Issue
- Dec 1987
- Veterans Administration (VA)
- EA
- Subscribe
- EA
 Similar Supply Items to 6515-00-176-5465
Similar Supply Items to 6515-00-176-5465


















 Freight Information 6515-00-176-5465
Freight Information 6515-00-176-5465
6515-00-176-5465 has freight characteristics.. 6515-00-176-5465 has a variance between NMFC and UFC when transported by rail and the description should be consulted.